

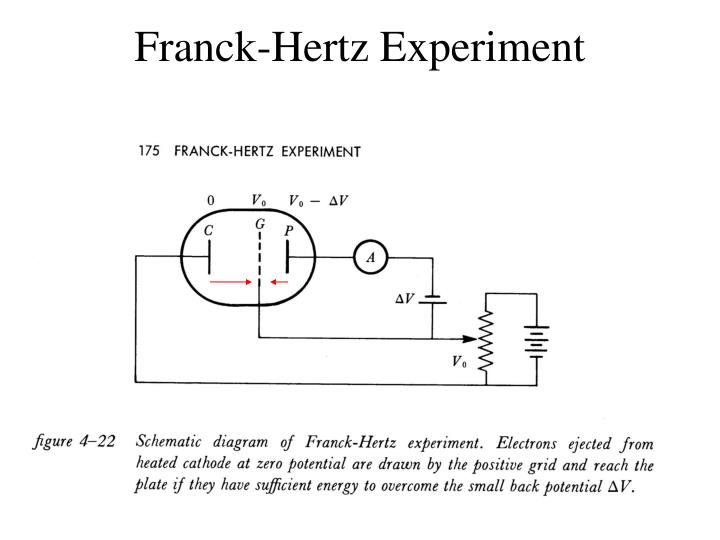
Moreover, this energy difference is the reason why the light is in the visible range. Furthermore, their de-excitement takes place by dropping to lower states at 16.57 and 16.79 eV. There are about ten peak electrons levels that lie in the range of 18.3 to 19.5 eV. Furthermore, when the accelerated electrons excite the electrons in neon to upper states, they de-excite in such a manner that there is the production of a visible glow in the gas region in which is occurring the excitation. One can clearly see the energy absorption from electron collisions in case of Neon gas. Again, an observation of a similar drop takes place at 9.8 volts.Again, there is an increase in the current as the increase in the voltage takes place to 9.8 volts.The dropping of the current is almost to zero at 4.9 volts.With the steady increase in the potential difference, there would be a steady increase of the current via the tube.The graph shows the following observations: The graph shows the dependence of the electric current that flows out of the anode and the electric potential present between the cathode and the grid. Also, the explanation of the experiment took place in terms of elastic and inelastic collisions between the electrons and the atoms of mercury. Moreover, the electric potential of an anode is slightly negative in comparison to the grid so that the electrons have the same kinetic energy as in the grid. The measurement of the electric current in the experiment is the result of the movement of the electrons from the grid to the anode. Furthermore, to draw emitted electrons, the voltage of the gird is positive with respect to the cathode. Three electrodes that are fitted include an electron-emitting hot cathode, a metal mesh grid, and an anode. Also, the fitting of the tube takes place with three electrodes. Furthermore, the drop of mercury was of the vapour pressure of 100 Pa. The original experiment made use of a heated vacuum tube of temperature 115 ☌. Naturwissenschaftliche Rundschau, 12, 237–239.The aim of the Frank-Hertz Experiment procedure is to demonstrate the concept of quantisation of the energy levels in accordance with the Bohr’s model of an atom. Schriften der Physikalisch-Ökonomischen Gesellschaft zu Königsberg., 38, 3–20. Philosophical Magazine, Fifth Series, 48, 547–567. On the Masses of Ions and Gases at Low Pressures. Philosophical Magazine and Journal of Science, Fifth Series, 46, 528–45. On the Charge of Electricity Carried by the Ions Produced by Röntgen Rays. Proceedings of the Cambridge Philosophical Society, 9, 243–4. Philosophical Magazine and Journal of Science, Fifth Series, 44, 293–316. Philosophical Magazine and Journal of Science, Fifth Series, 38, 418–420. Of the “Electron” or Atom of Electricity. Scientific Transactions of the Royal Dublin Society, IV, 563–680. On the Cause of Double Lines and of Equidistant Satellites in the Spectra of Gases. Cambridge, MA: Dibner Institute for the History of Science and Technology. Warwick, Histories of the Electron: The Birth of Microphysics (pp. Revue générale des sciences pures et appliquées, 19, 386–402. Rendiconti del circolo matematico di Palermo, 21, 129–176.
Comptes Rendus des Séances de l’Académie des Sciences, 121, 1130–34. Nouvelles propriétés des rayons cathodiques. CODATA Value: proton-electron mass ratio. National Institute of Standards and Technology. CODATA Value: electron charge to mass quotient. Proceedings of the Royal Society of London, 54, 438–461. A Dynamical Theory of the Electric and Luminiferous Medium. Die magnetische Ablenkbarkeit der Kathodenstrahlen und ihre Abhängigkeit vom Entladungspotential. British Journal for the History of Science, 241–276. Thomson and the ‘Discovery of the Electron’. Corpuscles, Electrons and Cathode Rays: J.


 0 kommentar(er)
0 kommentar(er)
